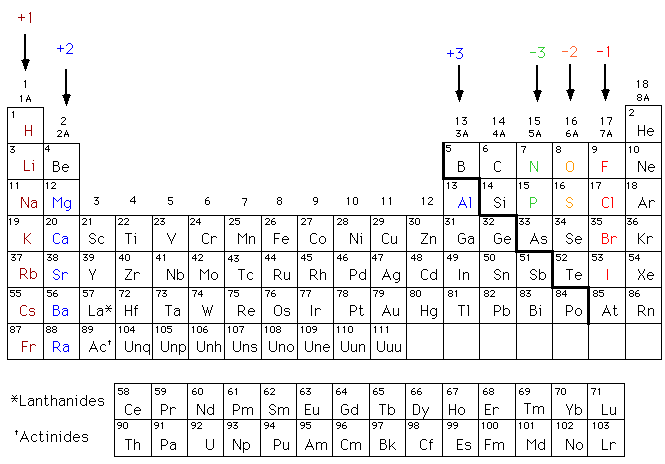
Metals Tend to Lose Electrons to Become Positive Ions
The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction. Mg Mg2 2 e-.
Since filled d or f subshells are seldom disturbed in a chemical reaction we can define valence electrons as follows.

. An ion ˈ aɪ ɒ n-ən is an atom or molecule with a net electrical charge. They can all be cut easily with a knife due to their softness exposing a shiny surface that tarnishes rapidly in air due to oxidation by atmospheric moisture and oxygen and in the case of lithium nitrogen. If we take a galvanic cell the anode is negative in nature and the electrons mostly move towards the external part of.
Generally at an anode negative ions or anions due to its electrical potential tend to react and give off electrons. Elements with three electrons or fewer in their outermost shells tend to be metals while those with five or more tend to be nonmetals. Access the answers to hundreds of The periodic table questions that.
Understand properties electronic configuration analogous behavior reactivity ionization energy. They are mixed with other metals to form an alloy that has the desired colour and strength. Electron Configurations of Ions As metals lose electrons to form cations and establish a noble gas configuration the electrons are lost from the valence shell first.
For example magnesium generally loses two electrons from its 3s subshell to look like neon. The electrons on an atom that are not present in the previous rare gas ignoring filled d or f subshells. The magnesium would lose two electrons becoming 2 charged and the oxygen would gain the two electrons becoming -2 charged in the process.
Most atoms have three different subatomic particles inside them. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton which is considered to be positive by conventionThe net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. Get help with your The periodic table homework.
3 Last example Mg and Cl. The alkali metals are all shiny soft highly reactive metals at standard temperature and pressure and readily lose their outermost electron to form cations with charge 1. The Periodic Table Questions and Answers.
Alkaline Earth Metals have their s-subshell filled with 2 valence electrons. These electrons then move up and into the driving circuit. If they gain electrons they will become an anion and carry a negative charge and if they lose electrons they will become a cation and carry a positive charge.
Unit 3 Periodic Table Remediation Group 2 elements like Be Mg are called alkaline earth metals. The positivenegative charge attraction this time 2-2 four times as much as the 1-1 of NaCl would hold the two ions together. Protons neutrons and electronsThe protons and neutrons are packed together into the center of the atom which is called the nucleus and the electrons which are very much smaller whizz around the outsideWhen people draw pictures of atoms they show the.
Polyatomic ions can be thought of in a very similar way to monoatomic ions in that they are ionized by either gaining or losing electrons so that they carry a charge. What are the parts of an atom. Those with exactly four electrons in the outermost shell behave as semimetals.
Elements that are nonmetals tend to gain electrons and. The lack of continuous FeO 6 octahedral network confines the conduction of electrons in the Fe-O-Fe resulting poor electronic conductivity of LiFePO 4 which greatly limit the application of LiFePO 4 in high rate conditions. It should point out that the arrangement of oxygen-metal ions in the hexagonal close-packing make the electron conductivity of the material quite low ie.
Metals are electropositive tending to lose their outermost electrons and become positive ions when they undergo chemical reactions.

Which Elements Tend To Lose Electrons What Charge Will They Become Enotes Com

Ionic Bonding A Love Story L A Cartoon Guide To Formation Of Ionic Bond L Science L Brar Scribbles Ionic Bonding Chemistry Classroom Chemistry Projects

Atoms Of Metals Tend To1 Lose Electrons And Form Negative Ions2 Lose Electrons And Form Positive Brainly Com

Why Do Metals Always Form Positive Ions Quora

3 433 Gostos 24 Comentarios Vanessa Adagio Studies No Instagram Managed To Get Stuff Done Today Notes Inspiration Study Notes School Organization Notes
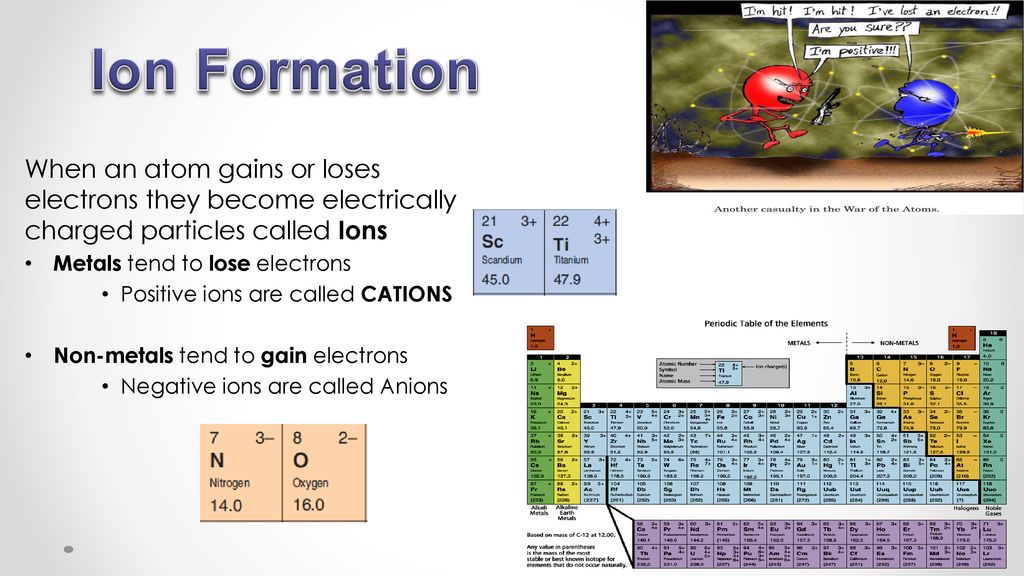
Ion Formation When An Atom Gains Or Loses Electrons They Become Electrically Charged Particles Called Ions Metals Tend To Lose Electrons Positive Ions Ppt Download

What Are Ions When Atoms Gain Or Lose Electrons They Become Ions This Means They Are No Longer Neutral Unit 3 Chemistry Ions And Ionic Bonding Ppt Download
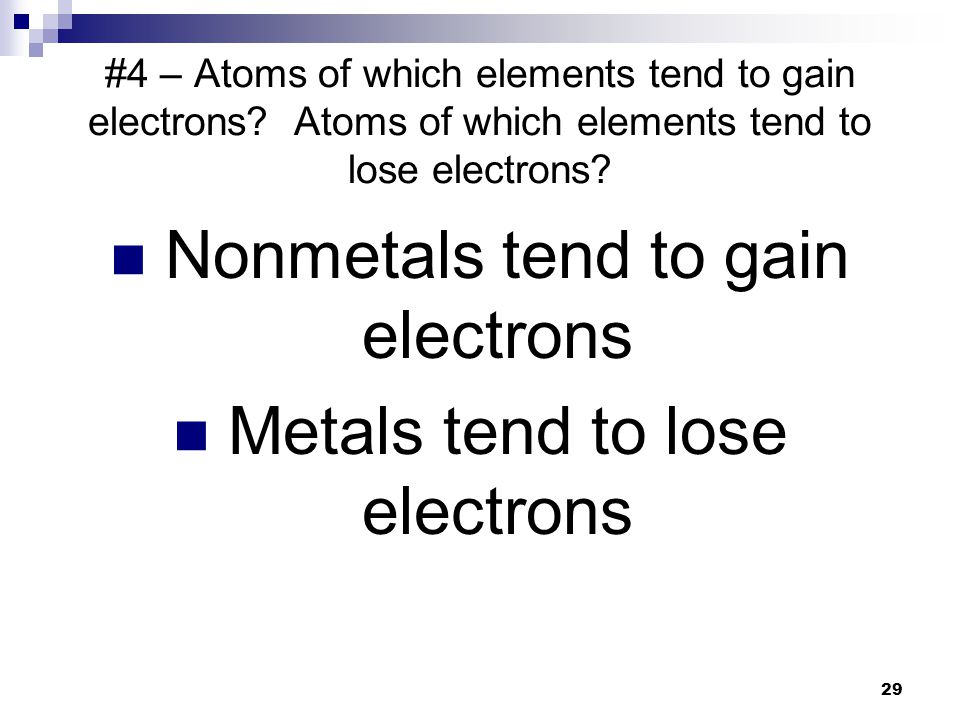
Ionic And Metallic Bonding Ppt Video Online Download

What Are Ions When Atoms Gain Or Lose Electrons They Become Ions This Means They Are No Longer Neutral Unit 3 Chemistry Ions And Ionic Bonding Ppt Download
Why Do Metals Always Form Positive Ions Quora

Ionic And Metallic Bonding And How 1 5 12 Objective To Review The Fundamentals Of Ions And Learn About Ionic Bonding Do Now Do The Hokey Pokey Turn Ppt Download

Chemical Bonding What It S All About Why Do Atoms Bond Together Why Should We Bother To Study Electron Configurations Helps Determine The Way Atoms Ppt Download
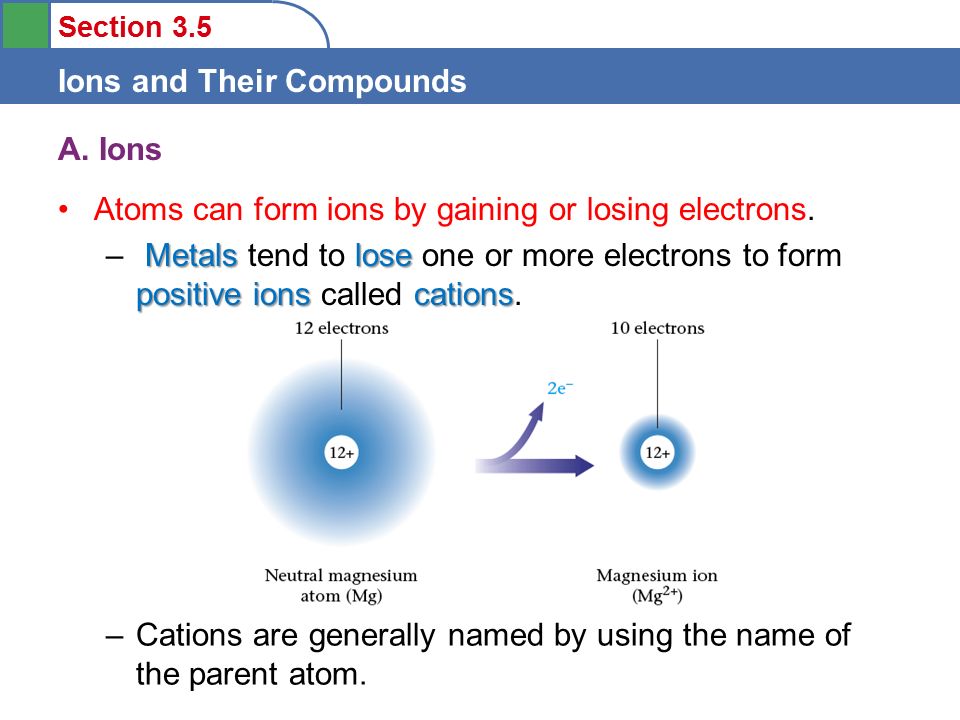
Objectives To Describe The Formation Of Ions From Their Parent Atoms Ppt Download

7 1 Compound Atoms And Ions Let S Review Look At These Elements Sodium Metal Fluorine Non Metal Neon Noble Gas Ppt Download

Science 10 Lesson 2 Bohr Models Ionic Bonding Student Review Draw A Model For Sulfur Atom Ppt Download
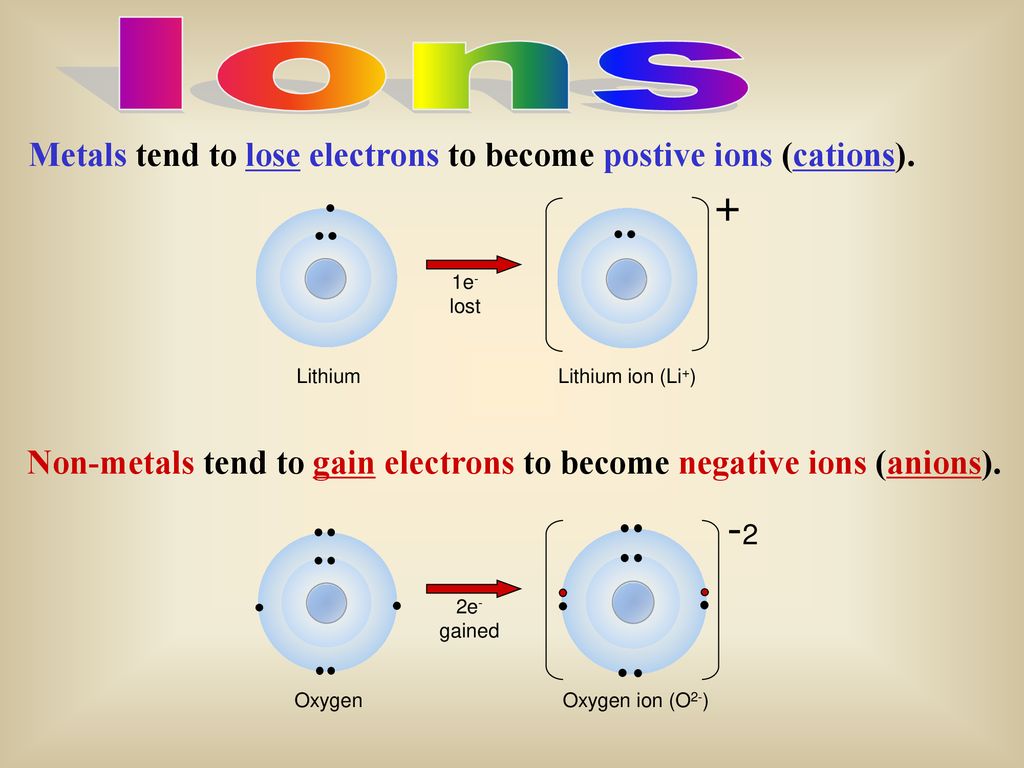
Comments
Post a Comment